Efficacy of Silicolgel in adults with irritable bowel syndrome subtypes IBS-D or IBD-M, Gastroenterology Research Practice & Practice, Crawford, Taylor, Young & Hatton 2023
In a recent trial, 84% of users showed a significant improvement in IBS symptoms.*
*Improvement in IBS symptoms severity over 4 weeks; Clinical Study published in Gastroenterology, Research and Practice, Dec 2023.
Silicolgel is supported by a wide range of evidence; clinical, in-vitro and literature.
Method:
In line with the recommended dosage, participants suffering Severe or Moderate IBS according to their IBS SSS Scores (Francis et al Irritable Bowel Syndrome Severity Scoring) underwent 4 weeks of treatment with Silicolgel and had assessments to monitor their progress after 1 week (visit 2; telephone), 2 weeks (visit 3; telephone) and 4 weeks (visit 4; in-clinic).
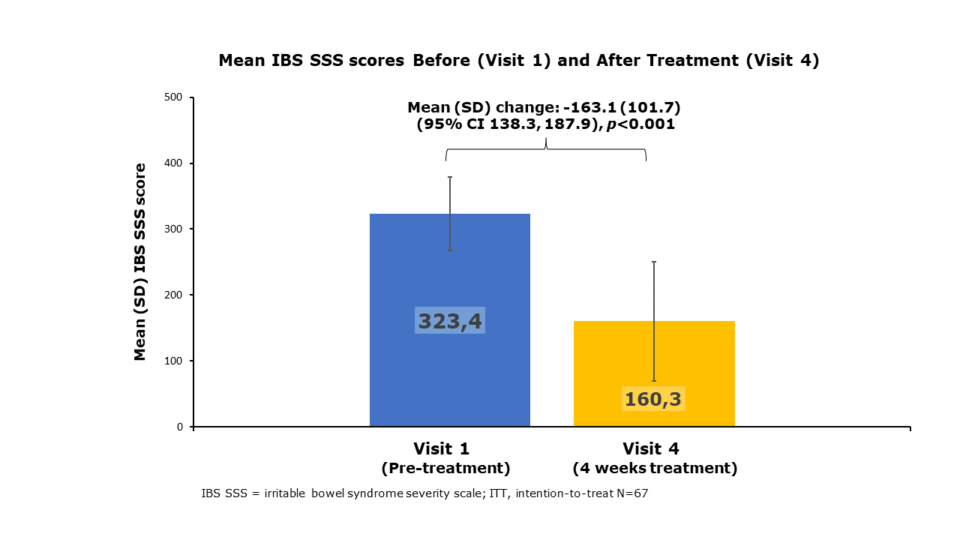
Mean IBS SSS scores of Visit 1 vs Visit 4
After 4 weeks of treatment, 83.6% of participants achieved the primary endpoint of a reduction in IBS SSS > 50, representing a clinically meaningful improvement in IBS
After 4 weeks of treatment, Mean IBS SSS score reduced from 323 to 160, a reduction of -163 representing a change from Severe to Mild IBS on the Francis Scale
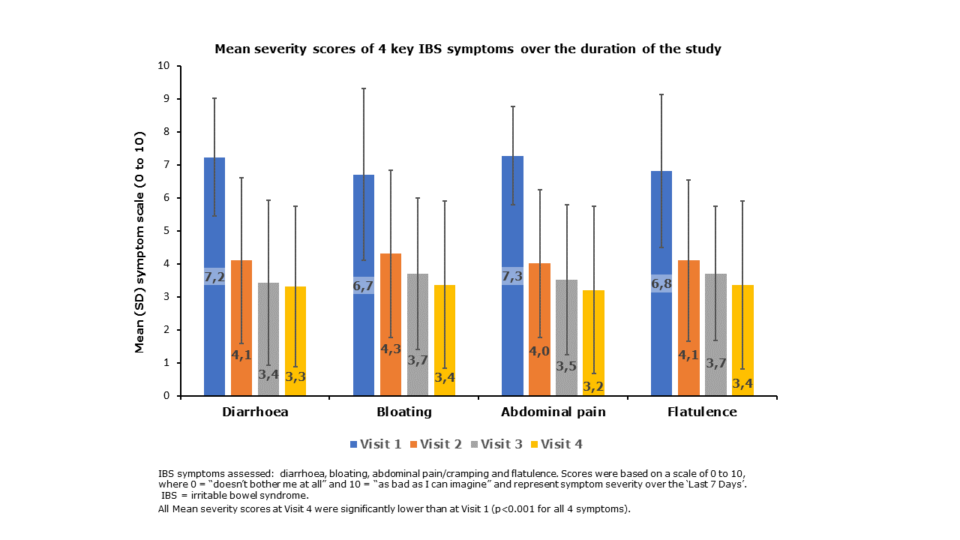
Reduction of key IBS symptoms over the duration of the study
Scores were based on a scale of 0 to 10, where 0 “doesn’t bother me at all” and 10 is “as bad as I can imagine” and represent symptom severity over the previous 7 days.
Significant improvements in symptom ratings were seen after 4 weeks of treatment for all 4 IBS symptoms
Symptom improvements were seen throughout the duration of the treatment and were noted for all symptoms from as early as after just one week of treatment
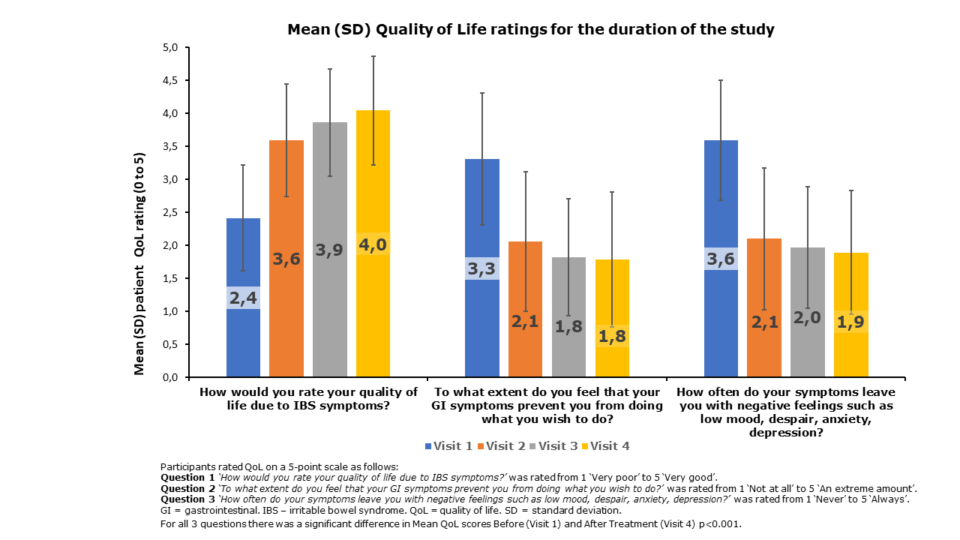
Improvement in the Quality of Life (QoL) scores over the duration of the study
Significant improvement was seen in all QoL ratings after 4 weeks of treatment
Progressive improvement in QoL were noted throughout the course of the study